About the Hand-2 study
Summary of the study
Dupuytren's contracture is a condition of the hand which causes one or more fingers to bend into the palm and prevents them from straightening fully. It is caused by a thickening of ligaments in the palm of the hand.
For people who have a troublesome, bent-up finger which has not been treated previously, usual NHS treatment is to either release the contracture by cutting it with a needle (Needle Fasciotomy), or to remove it (Limited Fasciectomy). Needle Fasciotomy requires injection of local anaesthetic into the hand. Limited Fasciectomy requires numbing the whole arm with local anaesthetic injected into the armpit or a general anaesthetic.
Both procedures are commonly performed across the UK; neither are new or experimental. We don’t know which of these treatments is best. If you take part in the study you will be randomly allocated to receive one of the two treatments.
If you take part you will be asked to complete some questionnaires over a two year period. These will be completed either in clinic or they will be sent to you via text/email /post to complete. You may also be asked if we can audio-record the discussions that you have with your doctor or other health professionals about taking part in the study. This is optional. Information about this will be provided separately.
We are also planning to interview some patients for up to an hour at a convenient time and through a preferred method. This will enable us to explore your experiences and acceptability on the treatment after surgery. Again, this is optional.
Further information:
What is the purpose of the study?
Dupuytren's contracture is a condition of the hand which causes one or more fingers to bend into the palm and prevents them from straightening fully. It is due to a thickening or tightening of ligaments in the palm of the hand. It is more common in men than women, and usually occurs in people who are aged over 50. The cause is unknown, although it does often run in families. The condition is benign and usually painless, but can lead to problems with activities such as washing, getting dressed, shaking hands, and using a computer keyboard.
Hand with Dupuytren’s contracture:
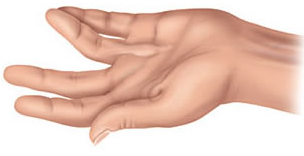
For finger(s) which have not been treated previously the usual NHS treatment for Dupuytren's contracture is to straighten the finger, either by cutting across the contracture, usually with a needle (called Needle Fasciotomy) or removing it (called Limited Fasciectomy).
Needle Fasciotomy
- Is performed as an outpatient in a hospital clinic.
- Local anaesthetic is used to numb part of your hand.
- A needle is used to divide the contracture, releasing the tightness to allow your finger/s to straighten. No cut is made in your skin, and no stitches are needed.
- For most people the hand recovers in 1 -2 weeks. The risk of significant complications is low (around 1%).
- The advantage is that recovery is quicker than with Limited Fasciectomy.
- Complications can be tearing the skin which delays healing and numbness in the finger.
- The Dupuytren’s contracture may come back to some extent in 8 out of 10 people within five years. Some of these 8 people will request further treatment.
Limited Fasciectomy
- Is performed as a day case in an operating theatre.
- Is usually done with you awake with the whole arm numbed (regional anaesthetic) but is sometimes done under general anaesthetic, which means you will be unconscious during the treatment.
- The surgeon will cut the skin in your hand and then remove the contracture and straighten the finger. You will have stiches to close the wound, which may need to be removed.
- For most people the hand will take 4 - 6 weeks to recover.
- The advantage is that the benefit of surgery is longer lasting than that of needle fasciotomy.
- It has a higher risk of complications than Needle Fasciotomy (around 5%). These can include problems with the wound healing, numbness in the finger and joint stiffness.
- The Dupuytren's contracture may come back to some extent in 3 out of 10 people within five years. Some of these 3 people will request further treatment.
We are conducting this study because although both treatments are commonly used to treat Dupuytren’s contracture, we do not know which one is best for patients and the NHS. Therefore, in this study patients with Dupuytren’s contracture will have either a Needle Fasciotomy or a Limited Fasciectomy.
- Why have I been invited to take part?
You have been invited to take part in this study as you may have Dupuytren’s contracture, and either Needle Fasciotomy or Limited Fasciectomy would be appropriate treatment for you.
It is up to you to whether or not you join the study. We will describe the study and go through this information sheet. If you agree to take part, we will then ask you to sign a consent form. You are free to withdraw at any time, without giving a reason. This would not affect the standard of care you receive.
What would taking part involve?
|
With your permission, we will inform your GP about your participation in this study.
If you take part in the study you will be put on the normal NHS waiting list for either (i) Needle Fasciotomy or (ii) Limited Fasciectomy for treatment of your Dupuytren’s contracture. As nobody knows which treatment is best, your treatment will be allocated using a process called randomisation. This means you will have an equal chance of having the Limited Fasciectomy or the needle fasciotomy. Randomisation is used as it creates groups of patients that are similar except for the treatment received. This will enable a fair comparison of the different treatments so we can assess at the end of the study which one is best for future patients. Neither you, the doctors or the research team can choose which group you will go in, as this could result in the groups being unequal and the findings unreliable.
We would like to follow you up for 2 years after your treatment to find out how you are doing. We will text/ email/post a questionnaire for your completion at 2, 3 and 4 weeks after your treatment asking about your ability to carry out normal daily activities with your hand, your hand function and your quality of life. We will ask you to complete these again six weeks after the treatment (a routine NHS clinic appointment) and at 6, 12 and 24 months either at an extra outpatient clinic appointment, or by texting/ emailing you the questionnaire to complete at home. We would also like you to take measurements of your finger using a SLiCK device at 6, 12 and 24 months. You will be shown how to do this and given an instruction manual at your initial clinic appointments. You will also have access an on-line video demonstration of how to do this.
As a token of appreciation, if you complete the week 12 questionnaire you will receive a £15 voucher and a further £15 voucher once you have completed the week 24 questionnaire (£15 per questionnaire).
We may also contact you up to 2 years after you have had treatment to hear your thoughts on the treatment and about taking part in the Hand-2 study. Discussion will cover hand function before and after treatment, expectations and experiences of the treatment and recovery, and views on taking part in the study. This part of the study is being coordinated by the University of Nottingham and the University of Bristol. The discussion (called a research interview) may last up to an hour, is likely to be over the telephone or through another preferred method and, if you agree it will be audio-recorded on an encrypted device. The discussions are optional and will be undertaken at a time that is convenient to you.
If you decide to take part in the study, you are free to withdraw at any time, without giving a reason. This will not affect the standard of care you receive.
You can indicate on the consent form if you are happy to be contacted in the future about taking part in further research studies.
You will be asked to sign a consent form to agree to take part in the study and to have your operation allocated by a process of randomisation and participation in the study. You will be asked to sign a separate consent form if you agree to a research interview.
If you decide to take part in the study, you are free to withdraw at any time, without giving a reason. This will not affect the standard of care you receive.
You can indicate on the consent form if you are happy to be contacted in the future about taking part in further research studies.
You will be asked to sign a consent form to agree to take part in the study and to have your operation allocated by a process of randomisation and participation in the study. You will be asked to sign a separate consent form if you agree to a research interview.
|
What are the possible benefits of taking part?
We cannot promise the study will help you but the information we get from this study may help us to treat people with Dupuytren’s contracture in future.
What are the possible disadvantages and risks of taking part?
|
Although both treatments are commonly used for Dupuytren’s contracture, we do not know which one is better for patients. That is why we are doing this study.
Both the treatments are available as routine NHS care, so there is no extra risk involved in receiving them as part of the study.
Taking part will mean spending some extra time to complete questionnaires however these can be completed via text or email.
There are no physical risks if you take part in the optional interviews. It is possible that talking about your feelings and other issues related to your diagnosis and treatment may cause anxiety. Please be aware that you are able to pause or finish the discussion with the researcher at any time.
|
What if there is a problem?
|
If you have concerns or questions about any aspect of this study, you should ask to speak to the local researchers. Their contact details are at the front of this sheet.
If any questions remain you can contact the main investigators of this study:
Name: Professor Tim Davis
Email: Tim.davis@nuh.nhs.uk
Or the Trial Manager at the Nottingham Clinical Trials Unit (NCTU), Tel: 0115 823 1578, Email: Hand2@nottingham.ac.uk If you remain unhappy and wish to complain formally, you can do this through the NHS Complaints Procedure via the Patient Advisory and Liaison Service (PALS) <insert Local PALS details>.
In the event that something does go wrong and you are harmed during the study, there are no special compensation arrangements. If you are harmed and this is due to someone’s negligence then you have grounds for a legal action for compensation but you may have to pay your legal costs. The normal NHS complaints mechanism will still be available to you.
|
What will happen if I don’t want to carry on with the study?
You are free to withdraw at any time, without giving any reason, and without your legal rights being affected. If you withdraw the information collected will not be erased and this information may still be used in the project analysis.
How will information about me be used?
|
We will follow ethical and legal practice and all information about you will be handled in confidence.
If you join the study, some of the data collected for the study may be looked at by authorised persons from the Nottingham Clinical Trials Unit (NCTU) at the University of Nottingham who are organising the research. They may also be looked at by authorised people from regulatory authorities and Nottingham University Hospitals NHS Trust to check that the study is carried out correctly. All will have a duty of confidentiality to you as a research participant and we will do our best to meet this duty.
All information which is collected about you during the course of the research will be kept strictly confidential, stored in a secure and locked office, and on a password protected database. Any written information about you which leaves the hospital will have your name and address removed (anonymised) and a unique code will be used so that you cannot be recognised from it. Your personal contact details will be sent to the Nottingham Clinical Trials Unit (NCTU) so they can send you questionnaires by post. A copy of your signed Informed Consent Form will be sent to Nottingham Clinical Trials Unit for review. This is to confirm that the study is being conducted in accordance with appropriate quality standards.
With your permission, we may use NHS Digital and other central UK NHS bodies to help us keep in touch with you and your health status. We will have confidentiality and security agreements in place to make sure your details are dealt with in the strictest confidence. In addition, the anonymised information collected about you may be used to support other research in future and may be shared with other researchers.
Your personal data (address, telephone number) will be kept after the end of the study so that we are able to contact you about the findings of the study (unless you advise us that you do not wish to be contacted). During this time all precautions will be taken by all those involved to maintain your confidentiality, only members of the research team will have access to your personal data.
Your name and telephone number will be held by Nottingham Clinical Trials Unit (NCTU) (University of Nottingham) and Esendex (text messaging provider) and will be used to contact you by text message. Also University of Bristol will need to have your name and contact details to contact you to arrange a research interview if you agreed to this.
Researchers at the Nottingham Clinical Trials Unit (part of the University of Nottingham) will need to use information from you, your medical records and your GP for this research project.
This information will include your initials, NHS number, name and contact details. The researchers will use this information to do the research or to check your records to make sure that the research is being done properly.
People who do not need to know who you are will not be able to see your name or contact details, your data will have a code number instead.
All information about you will be kept safe and secure.
Once the study has finished, some of the data will be kept so the results can be checked and you can be told what happened in the study (unless you tell us you do not want to know). Reports will be written in a way so that no-one can work out that you took part in the study.
Research interviews: The audio-recordings from the research interviews will be securely transferred from the encrypted audio-recorder to the University of Bristol and stored in a password protected drive maintained by the University. Recordings will be transcribed (written up word for word) by University of Bristol employees or University approved transcription services as soon as possible after each recording has been received. If an approved external transcription service is used, the transfer of recordings and transcripts will adhere to the secure transfer of recordings/transcripts procedure specified by the University. Transcripts will be labelled with a study-assigned participant number, edited so that you cannot be identified (anonymised) and stored securely adhering to the University’s data storage policies. Recordings and anonymised transcripts will be securely retained by the Universities of Bristol and Nottingham for at least five years after study closure. After five years the recordings will be destroyed but the anonymised transcripts will be retained for future research with your permission. Extracts of text (quotes) may be used in reports and publications, but you will not be identified from them. Anonymised transcripts may be made available to other researchers (including those outside of the Universities) who secure the necessary approvals required for controlled access for purposes not related to this study, subject to your permission.
|
What are your choices about how your information is used?
You can stop being part of the study at any time, without giving a reason, but we will keep information about you that we already have.
We need to manage your records in specific ways for the research to be reliable. This means that we won’t be able to let you see or change the data we hold about you.
If you give us your permission we may keep your contact details so we can get in touch if there is any relevant future research to do with your condition that you may be interested in taking part in. You will also have the option to take part in future research using your data saved from this study. If you do not wish for your contact details to be kept for a copy of the study results to be sent to you or to be contacted about future research, these will also be disposed of securely at the end of the study.
Where can you find about more about how your information is used?
You can find out more about how we use your information:
Who is organising and funding this study? How has it been approved?
The study is being organised by the Nottingham University Hospitals NHS Trust (the Sponsor) and coordinated by the Nottingham Clinical Trials Unit (NCTU). The funding for the study is provided by the Health Technology Assessment Programme, which is part of the National Institute for Health Research (the research arm of the NHS).
All research in the NHS is looked at by an independent group of people, called a Research Ethics Committee, to protect your interests. This study has been reviewed and given favourable opinion by London-Chelsea Research Ethics Committee.
Patients who have previously been treated for Dupuytren’s contracture have helped us plan and design this study. Patients’ representatives are also involved in the teams that oversee the running of the study.
What if relevant new information becomes available?
Sometimes we get new information about your treatment/ during the study. If this happens your research doctor will tell you about this new information and discuss whether you should continue in the study. If you decide not to carry on, your research doctor will make arrangements for your care to continue as normal. If you decide to continue in the study he/she may ask you to sign a new Informed Consent Form.
What happens at the end of the study?
When the study ends, your healthcare will continue as normal. If you withdraw from the study, we will need to keep and use the data collected up to your withdrawal. At the end of the study the results will be published in scientific medical journals and presented at conferences. You will not be identified in any publication. We will send you a newsletter with a summary of the study findings, unless you ask us not to.